Is it possible to acquire salmonellosis from medications?
Salmonella is one of the most well-known microorganisms globally. Each year, health authorities conduct various campaigns to raise public awareness and prevent infections caused by these Gram-negative bacteria, including warnings about the importance of not washing raw chicken. However, little attention is paid to the risks posed by Salmonella in non-food products, such as medications.
As previously indicated in other articles, microbiological control in the pharmaceutical industry is divided into two main categories: sterile and non-sterile products. In the case of sterile products, one of the methods used to ensure sterility is the Media Fill Test, which simulates the manufacturing process and encompasses all phases of production.
Regarding non-sterile medications, the primary purpose is to prevent contamination of the final product and to maintain low levels of microorganisms during the manufacturing process. By implementing these two measures, it is possible to manage the overall biological load and reduce the likelihood of contamination, thereby decreasing the risk to consumers.

One key indicators in the pharmaceutical industry is Salmonella, as this pathogen can lead to severe gastrointestinal diseases and even systemic infections. It’s important to recognize that the end users of medications are often significantly immunocompromised, which can lead to more severe symptoms and complications from infections. Additionally, as an indicator organism, the presence of Salmonella is useful for identifying problems related to production control and shortcomings in Good Manufacturing Practices (GMP).
It is well understood that ISO standards do not include drug analysis; instead, this falls under the jurisdiction of Pharmacopoeias, with each country or region having its own version. However, the European (EP), American (USP), and Japanese (JP) Pharmacopoeias are frequently referenced.
According to the European Pharmacopoeia, Salmonella should not be present in non-sterile products. Nevertheless, a study conducted in Europe revealed that nearly 2% of the 1,285 samples examined, primarily comprising natural origin drugs (such as plant extracts), failed to meet the required microbiological standards.
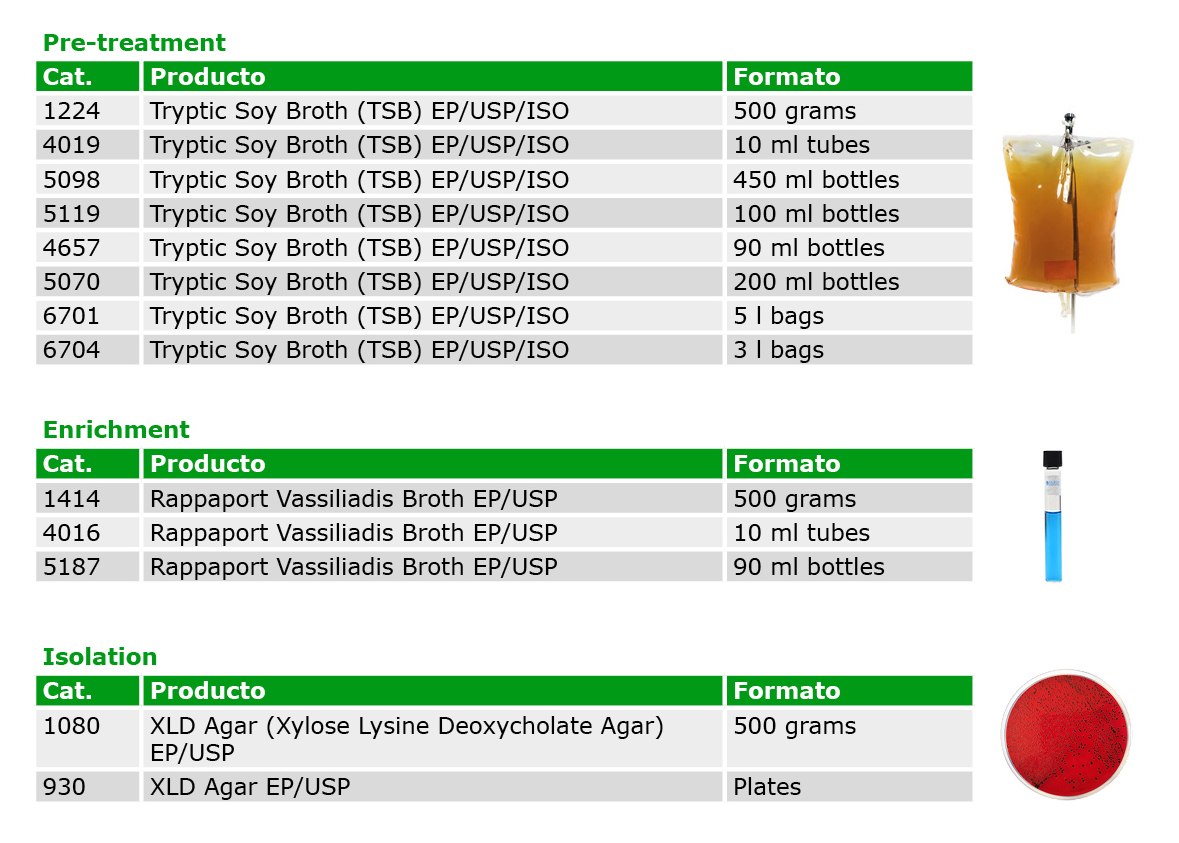
Traditionally, analyzing for Salmonella required the use of several media (such as Lactose, Brilliant Green Agar, Bismuth Sulfite Agar, Selenite Cystine Broth, Tetrathionate Broth, and TSI Agar), varying by Pharmacopoeia. However, following the harmonization of the EP/USP/JP, a single standardized method can now be employed, utilizing only three highly selective media for the detection of this pathogen.
Asiagel offers the necessary media in various formats for enhanced efficiency and convenience.